
(Photo provided by Sinopharm)
Forty-three million shots of the COVID-19 vaccine developed by Chinese company Sinopharm have been administrated worldwide, with 34 million in China and the country's yearly production capacity could reach 4 billion by the end of 2022, China Central Television (CCTV) reported Saturday.
China has issued emergency approval for clinical trials for 16 COVID-19 vaccines candidates with six in Phase III, according to the National Medical Products Administration.
Eighteen production lines for COVID-19 vaccines have been established. The nation's annual COVID-19 vaccines production capacity is expected to reach 2 billion doses by the end of 2021 and 4 billion by the end of 2022, Feng Duojia, president of the China Vaccine Industry Association told CCTV.
Four billion would cover 40 percent of world demand, Feng previously told the Global Times.
The Global Times also learned from another Beijing-based producer Sinovac Biotech on Wednesday that the company's second production line is now making more than one million doses a day.
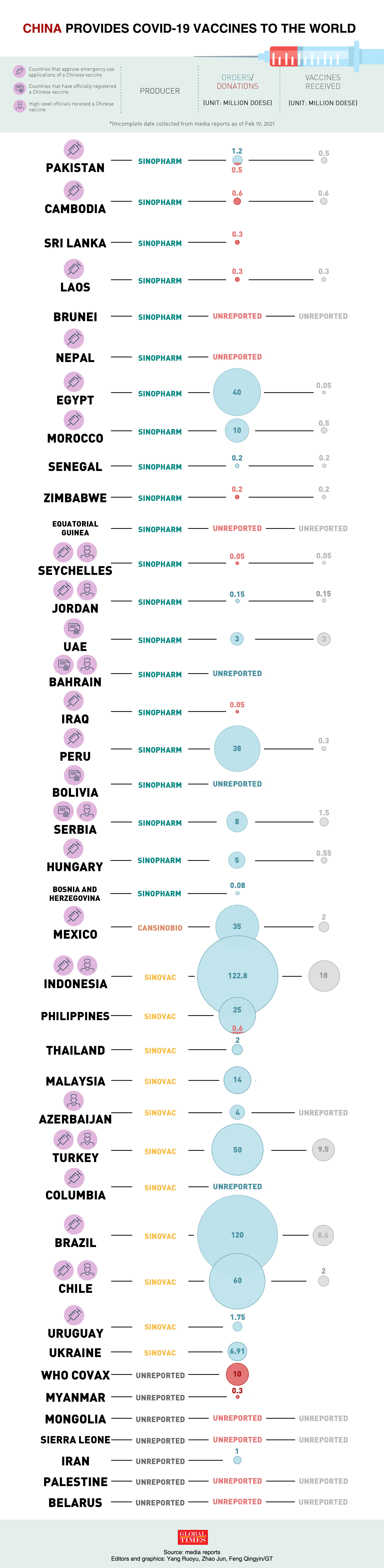
According to the Chinese Foreign Ministry, China has provided vaccines to 53 developing countries and is exporting vaccines to 22 countries. Eight foreign leaders have been vaccinated with a Chinese vaccine.
China has also approved 22 drugs for COVID-19 and its indications for clinical trials; Twenty-four million coronavirus test kits of 54 different types are being produced each day, according to CCTV.
(Graphic: Global Times)


