JAKARTA, Jan. 11 (Xinhua) -- Indonesia's food and drug authority BPOM on Monday issued the emergency use authorization for Sinovac's COVID-19 vaccine as the country will begin administering doses of COVID-19 vaccine on Wednesday.
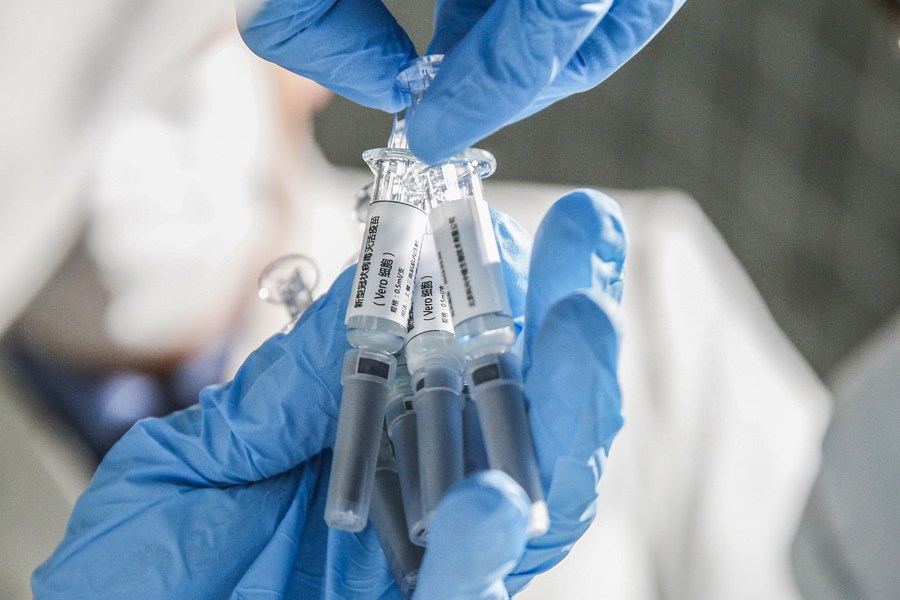
A staff member displays samples of the COVID-19 inactivated vaccine at Sinovac Biotech Ltd, in Beijing on March 16, 2020. (Photo: Xinhua)
At a press conference, BPOM chief Penny Lukito said that the COVID-19 vaccine produced by the Chinese biopharmaceutical company Sinovac Biotech, which had been tested in the phase 3 at Padjadjaran University, met the safety standards set by the World Health Organization.
The evaluation results of the supporting safety data obtained from the third phase of clinical studies in Indonesia, Brazil and Turkey are overall safe with the incidence of mild to moderate side effects, she said.
Indonesian Health Minister Budi Gunadi Sadikin said Monday that the 15 million doses of the raw materials from Sinovac will be received on Tuesday.
As of Monday afternoon, there have been more than 800,000 confirmed cases of COVID-19 reported in Indonesia. The coronavirus epidemic has claimed more than 24,000 lives across the archipelago, according to the Health Ministry.


