For the first time, a pig kidney has been transplanted into a human without triggering immediate rejection by the recipient's immune system, a potentially major advance that could eventually help alleviate a dire shortage of human organs for transplant.
The procedure done at the New York University (NYU) Langone Health, involved the use of a pig whose genes had been altered so that its tissues no longer contained a molecule known to trigger almost immediate rejection.
The recipient was a brain-dead patient with signs of kidney dysfunction whose family consented to the experiment before she was due to be taken off of life support, researchers said.
For three days, the new kidney was attached to her blood vessels and maintained outside her body, giving researchers access to it.
Test results of the transplanted kidney's function "looked pretty normal," said transplant surgeon Dr Robert Montgomery, who led the study.
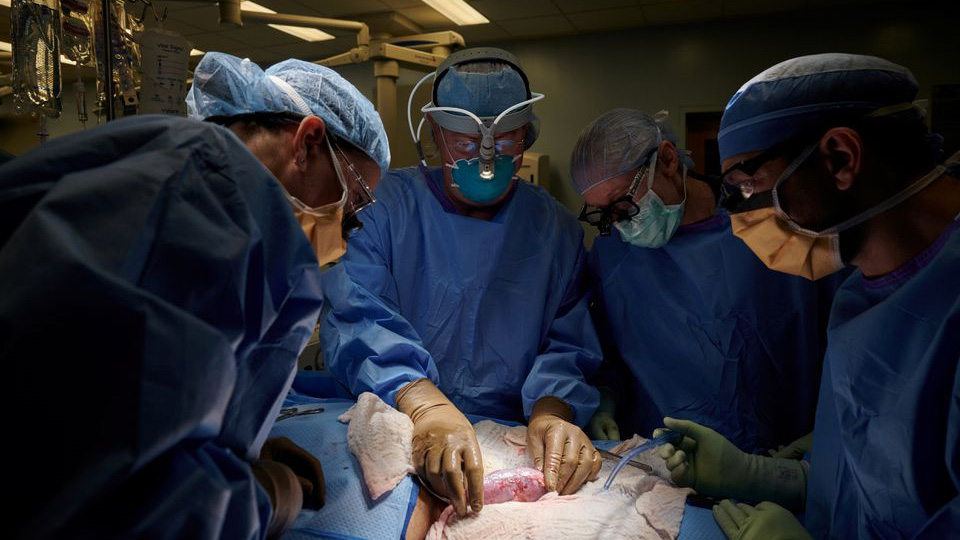
The surgical team examines the pig kidney for any signs of hyperacute rejection, as the organ was implanted outside the body to allow for observation and tissue sampling during the 54-hour study period, at NYU Langone, New York, U.S. (Photo from CGTN)
The kidney made "the amount of urine that you would expect" from a transplanted human kidney, he said and there was no evidence of the vigorous and early rejection seen when unmodified pig kidneys are transplanted into non-human primates.
The recipient's abnormal creatinine level – an indicator of poor kidney function – returned to normal after the transplant, Montgomery said.
In the United States, nearly 107,000 people are presently waiting for organ transplants, including more than 90,000 awaiting a kidney, according to the United Network for Organ Sharing. The waiting period for a kidney may average three-to-five years.
Researchers have been working for decades on the possibility of using animal organs for transplants, but have been stymied over how to prevent immediate rejection by the human body.
Montgomery's team theorized that knocking out the pig gene for a carbohydrate that triggers rejection – a sugar molecule, or glycan called alpha-gal – would prevent the problem.
The genetically altered pig was approved by the U.S. Food and Drug Administration in December 2020, for use as food for people with a meat allergy and as a potential source of human therapeutics.
Medical products developed from the pigs would still require specific FDA approval before being used in humans, the agency said.


